The Synergistic Potential of Cannabis
Introduction
The cannabis plant has been used for medical, spiritual, and recreational purposes for thousands of years. Today, cannabis is increasingly accepted as a legitimate medicinal option, with pharmaceutical cannabinoids like Tetrahydrocannabinol (THC) and Cannabidiol (CBD) demonstrating therapeutic effects for conditions ranging from chronic pain to epilepsy.
Moreover, over 500 distinct compounds have been identified in the cannabis plant, including a diverse array of terpenes that drive distinctive aromas, flavors, colors, and effects. The concept of the “Entourage Effect” suggests that the interactions between terpenes, cannabinoids, and other cannabis components are responsible for the unique medicinal properties of the whole plant2.
Specifically, terpenes may enhance cannabinoid activity in the body’s endocannabinoid system to provide synergistic health benefits unattainable through isolated components alone.
This white paper reviews current scientific evidence on cannabis terpenes, cannabinoids, the Entourage Effect, and their implications for human health and medicine. Critical knowledge gaps are identified, and recommendations made for future research directions to harness the full therapeutic potential of this remarkable plant.
Cannabinoids
Over 120 unique phytocannabinoids have been identified in cannabis. These specialized secondary metabolites are biosynthesized and accumulated predominantly within the glandular trichomes – the dense hair-like secretory structures protruding from the surface of female cannabis flowers.
Trichomes provide a physiological reaction vessel housing the enzymes, substrates, cofactors, transporters, and reaction conditions required for cannabinoid assembly. They later function as storage vessels for the synthesized hydrophobic cannabinoids in the trichome heads.
Reflecting ancestral selection pressures, modern cannabis strains direct a sizable portion of metabolic energy toward cannabinoid production — up to 25% dry weight of mature trichomes consists of cannabinoids. This extraordinary biosynthetic investment suggests important ecological roles, which we are only beginning to unravel. The two most studied cannabinoids include THC (Tetrahydrocannabinol) and CBD (Cannabidiol):
∆9-tetrahydrocannabinol (THC)
THC is the primary intoxicating compound in cannabis. However, evidence suggests THC also has analgesic, antiemetic, neuroprotective, anti-inflammatory, and antitumor properties3.
THC acts as a partial agonist at the cannabinoid 1 (CB1) and cannabinoid 2 (CB2) G-protein coupled receptors, responsible for the psychoactive effects as well as potential therapeutic benefits.
___________
#An Agonist is “one that activates”, as in capable of activating receptors. Conversely, an Antagonist will not.
Cannabidiol (CBD)
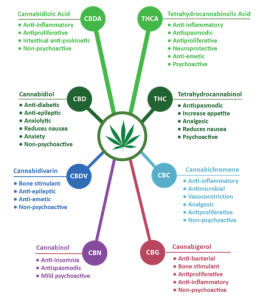 In contrast to THC, CBD is non-intoxicating. CBD interacts with myriad molecular pathways and receptor systems distinct from the cannabinoid receptors, including Transient Receptor Potential (TRP) channels, Peroxisome Proliferator Activated Receptors (PPARs), and others4 . Through these targets, CBD demonstrates analgesic, anti-anxiety, antiemetic, anti- inflammatory, and neuroprotective properties.
In contrast to THC, CBD is non-intoxicating. CBD interacts with myriad molecular pathways and receptor systems distinct from the cannabinoid receptors, including Transient Receptor Potential (TRP) channels, Peroxisome Proliferator Activated Receptors (PPARs), and others4 . Through these targets, CBD demonstrates analgesic, anti-anxiety, antiemetic, anti- inflammatory, and neuroprotective properties.
Other Cannabinoids
Over 100 less-studied cannabinoids have been identified in cannabis alongside THC and CBD. Though not as thoroughly characterized, many of these minor cannabinoids demonstrate promising pharmacological activities and unique mechanisms of action, likely contributing significantly to the Entourage Effect. Among the most studied are:
Tetrahydrocannabivarin (THCV)
THCV behaves as a neutral CB1 antagonist, blocking the receptor at higher doses while activating it at lower doses. THCV also acts as a CB2 partial agonist. Through these mechanisms, THCV suppresses appetite and reduces body weight gain in obese rodent models6. Given the link between the endocannabinoid system and metabolic regulation, THCV shows promise as a novel treatment for metabolic disorders.
Cannabinol (CBN)
Oxidative degradation converts THC into CBN over time. CBN has approximately 10% of the affinity^^ for CB1 compared to THC, contributing mildly intoxicating effects. However, CBN modulates ion channels distinct from the cannabinoid receptors, acting as a potent agonist at TRPV2 channels and antagonist at TRPM8 channels^. Through these mechanisms, CBN demonstrates unique anti-inflammatory, analgesic, anticonvulsant, and sedative properties7.
Cannabichromene (CBC)
Unlike THC, CBD, and CBN, CBC does not significantly bind the cannabinoid receptors. However, CBC regulates endocannabinoid tone by inhibiting anandamide uptake to boost signaling at CB1 and CB2 receptors8. Through this mechanism, alongside effects on TRP channels, CBC exerts modest analgesic and antidepressant effects in rodent models. Conversion of precursor CBCa by light or heat generates CBL, a degradation product of CBC.
Cannabigerol (CBG)
Serving as the parent compound in cannabis biosynthesis, most CBG converts to downstream cannabinoids like THC and CBD. But CBG elicits effects in its own right by inhibiting endocannabinoid uptake, in addition to GABAA+ and α2 adrenergic receptor activity9.
CBG demonstrates promising neuroprotective properties in preclinical models of Huntington’s and Parkinson’s diseases. Together with the major cannabinoids THC and CBD, these minor constituents form a dynamic web of bioactive compounds, interacting synergistically to contribute to cannabis pharmacology and theEntourage Effect.
___________
+ GABAA, or GABAA receptors are the major inhibitory neurotransmitter receptors in the mammalian brain. Alpha-2 receptors
are implicated in diverse physiological functions in the heart, and presynaptic alpha-2 receptors inhibit the release of
norepinephrine and other neurotransmitters in both the central and peripheral nervous systems.
^ TRPV2 and TRPM8 are TRP ion channels that sense heat and cold respectively.
^^ Affinity means the ability to interact with receptors.
Terpenes

Terpenes constitute the largest class of phytochemicals in cannabis. Responsible for the characteristic aromas of plants, over 200 terpenes have been identified in cannabis.
The 10 most abundant terpenes in cannabis are Myrcene, Limonene, Trans-caryophyllene, Linalool, Humulene, Pinene, Eucalyptol, Terpinolene, Fenchol, and Camphene.
Beyond aroma, terpenes play vital ecological roles for plants, including direct and indirect defense against herbivores. Several lines of evidence also suggest terpenes have pharmacological relevance for humans at physiological concentrations 11.
Biosynthesis
The huge structural diversity of the terpenome arises from the incredible capacity of enzymes. Using the universal five-carbon building blocks IPP (isopentenyl pyrophosphate) and DMAPP (dimethylallyl pyrophosphate), nature performs impressive feats of combinatorial chemistry. Through positively charged carbon atom cascades, terpene synthases drive the assembly of over 40,000 unique skeletons underpinning the entire terpenoid class. These backbone scaffolds then undergo tailoring by secondary enzymes, creating additional layers of complexity.
The resulting terpenoid metabolic grid underlies a vast assembly of plant natural products.
For instance, the monoterpene Pinene, common in conifers (pine trees), arise from slight divergence during ionization of their common precursor GPP (geranyl pyrophosphate).
The terpene synthase Pinene Cyclase generates an initial carbocation++ intermediate, which through loss of a proton forms the Alpha- or Beta-Pinene isomers.
Other monoterpenes like Limonene arise via related cyclisation chemistry.
This combinatorial generates terpene backbones spanning hemiterpenes (5 Carbon atoms) to tetraterpenes (40 Carbon atoms). Further tailoring by decorating enzymes imparts the vast chemical feature space of over 80,000 characterized terpenoids, with new structures reported daily. This unparalleled molecular abundance underlies the functional plasticity of terpenes across ecology and physiology.
_________
++ A carbocation is an ion with a positively charged carbon atom.
Pharmacological Relevance
In plants, terpenes mediate far more than fragrance. These compounds play pivotal ecological roles in growth regulation, development, photosynthesis, and defense against environmental threats. For example, monoterpenes can raise leaf temperatures in tropical vines, while the triterpene Saponins deters insect feeding. As the largest class of natural products known to science, terpenoid metabolic pathways provide enormous adaptability, better enabling plant survival.
Intriguingly, terpenes also influence human physiology. Both clinical and preclinical studies reveal anti-inflammatory, analgesic, anxiolytic, antidepressant, anticancer, and other bioactivities, partly supporting traditional plant medicine systems. Effects manifest from modulating receptors, ion channels, neurotransmitters, enzymes, and transporters.
The bioactivity of terpenes likely arises from effects on receptor signaling pathways and ion channels##. For instance, Linalool contributes to glutamatergic@ signaling through NMDA and AMPA receptors**, while the Beta-Caryophyllene directly activates cannabinoid CB2 receptors. Such interactions likely constitute initial triggers prompting broader cell signaling cascades and integrated systemic responses.
Similarly, Beta-Caryophyllene easily binds to the cannabinoid CB2 receptor. Modulation of such molecular targets by terpenes, sometimes at very small concentrations, probably contributes to the overall therapeutic effects of whole plant cannabis preparations.
The Entourage Effect
While over 100 terpenes have been identified in cannabis, evidence suggests those in highest abundance such as Myrcene and Limonene drive the majority of observed effects13.
Moreover, cannabis contains numerous additional components, including flavonoids, alkaloids, and other specialized metabolites with poorly understood function. The Entourage Effect concept proposes that dynamic crosstalk between these components enhances therapeutic outcomes beyond the expected additive contributions of isolated constituents14.
___________
** The N-methyl-D-aspartate (NMDA) receptor is a glutamate receptor found in neurons. The NMDA receptor is one of three
types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors.
## Ion channels are membrane proteins that allow ions to pass through them. They have various roles in cell signaling,
transport, and excitation.
@ Glutamatergic means “related to glutamate”. A glutamatergic agent is a chemical that directly modulates the excitatory
amino acid system in the body or brain.
In other words, the medicinal impact of whole plant cannabis exceeds the sum of its parts. Several lines of evidence support this theory, including both pre-clinical and clinical.
Preclinical Evidence
Preclinical rodent studies reveal profound Entourage Effects between the abundant terpene Myrcene and intoxicating cannabinoid THC. Co-administration of Myrcene increases THC brain uptake and tissue retention through increased permeability of the blood-brain barrier.
In tandem, Myrcene slows development of tolerance to repeated THC exposure through mechanisms yet unknown. These pharmacokinetic and pharmacodynamic interactions substantially enhance THC potency and duration of action, demonstrating robust synergy.
Additional terpenes also influence cannabinoid activity in nuanced ways. For instance, Limonene alters THC binding affinity and efficacy at CB1 receptors differently across rodent brain regions15. Limonene may drive CB1 interactions into distinct functional conformations through molecular binding mechanisms.
Relatedly, Linalool amplifies temperature decreases from THC administration, suggesting hypothermic synergy16. Intriguingly, many terpene effects manifest only in combination with cannabinoids like THC, supporting interdependence underlying entourage interactions.
Current preclinical data indicates a dynamic crosstalk between cannabis components. Indeed, the reported differences in experiential outcomes between medical cannabis chemovars likely arise from complex synergies between terpenes and cannabinoids. Isolated or synthetic cannabinoids simply cannot replicate the multidimensional therapeutic potential of whole plant formulations. Further inquiry into the polymodal crosstalk underlying cannabis synergy promises optimized treatment modalities.
Clinical Evidence
Clinical comparisons between isolated cannabinoids and whole plant preparations provide further evidence for the Entourage Effect. For neuropathic pain, Nabiximols (Sativex) is an oromucosal spray formulation containing a 1:1 ratio of THC and CBD, alongside other plant components like terpenes.
In Multiple Sclerosis and advanced cancer patients with treatment-resistant pain, Nabiximols show greater symptomatic relief for neuropathy vs. THC alone17.
Furthermore, Myrtol Standardized Essential Oil from the common Myrtle plant, Myrtus Communis, enhanced the effectiveness and tolerability of THC for cancer pain18. Emerging clinical data has also connected specific cannabis terpene profiles with subjective outcomes like analgesic and anxiolytic (anti-anxiety) efficacy.
Collectively, human studies corroborate interactions between cannabinoids and terpenes underlying observed differential responses to medical cannabis preparations.
Molecular Mechanisms and Signaling Pathways
Terpenes modulate critical cell signaling pathways, altering gene expression, protein function, metabolism, and physiology. Advanced “omics” technologies now enable determination of global molecular impacts. For example, Transcriptomics* reveals gene activity changes, Proteomics measures protein levels, and Metabolomics profiles cell metabolites. Together, integrating multi-omics datasets provides a blueprint of terpene cell regulation 19.
Within cells, terpenes modulate intricate signaling cascades that govern fundamental processes like metabolism, stress response, proliferation, and survival. Advanced ”- omics” technologies now allow comprehensive mapping of these molecular perturbations.
Transcriptomics* reveals terpene-induced gene expression changes. Proteomics profiles global protein level fluctuations. Metabolomics highlights altered cellular small molecule repertoires. Together, synergistic multi-omics analysis provides an integrated blueprint of terpene bioactivity.
___________
*Transcriptomics is the study of the ‘transcriptome,’ a term now widely understood to mean the complete set of all the
ribonucleic acid (RNA) molecules (called transcripts) expressed in some given entity, such as a cell, tissue, or organism.
In parallel, focused approaches definitively identify direct terpene-protein interactions. Activity-based protein profiling quantifies binding across the proteome through labeled chemical probes. Pull-down assays@@ also definitively link terpenes to protein targets.
In the laboratory, terpenes are immobilized on a fixed substrate material. Following cell incubation, remaining proteins are identified by mass spectrometry, confirming specific binding partners. Microarray (lab-on-a-chip) platforms composed of silicon chips have the ability to detect thousands of proteins simultaneously.
Terpene derivatives coat microscope slides, which capture native proteins from solutions. Binding events at each spot are quantified by techniques such as Fluorescence Imaging, highlighting targets for further investigation. Bioinformatics tools like Molecular Docking& may also predict potential terpene binding sites and affinities on protein structures.
Understanding the intricate molecular interplay that underlies terpene pharmacology will undoubtedly uncover novel therapies and opportunities for more personalized, precision botanical medicine.
Quantitative Structure-Activity Relationships
Over 80,000 unique terpene structures have been discovered in nature thus far, with new compounds reported nearly every day. This ever-expanding molecular repertoire, referred to collectively as the terpenome, represents the largest and most diverse class of natural products on the planet.
Underlying terpene diversity is the incredible pliability of their isoprenoid scaffolds. Slight modifications of these basic building blocks — tweaking stereochemistry, appending functional groups, forming cyclizations, or altering oxidation states — profoundly reshapes terpene geometry and chemistry. In turn, these structural alterations drastically influence pharmacological potency, bioavailability, and target selectivity.
For example, Alpha-Pinene increases acetylcholinesterase&& activity, while its isomer Beta-Pinene inhibits the enzyme. Similarly, Limonene blocks cell cycle progression in cancer models, yet its derivatives Perillyl Alcohol and Perillic Acid trigger apoptosis (programmed cell death)20.
Myrcene relaxes muscles, Linalool is sedative, and Geraniol anti-inflammatory, though all share the same chemical backbone structure. Research clearly shows that terpene scaffolds demonstrate near-infinite functional tunability.
Predicting Terpene Bioactivity “In Silico”
“In Silico” refers to the use of computer simulation to determine drug viability. Leveraging today’s growing computational power, Quantitative Structure-Activity Relationship (QSAR) computational software models enable prediction of terpene bioactivity based solely on chemical structures.
_________________
*A pull-down assay is an in vitro (external of an organism, as in a petri dish) technique used to detect physical interactions
between two or more proteins and is an invaluable tool for confirming a predicted protein-protein interaction.
* Molecular docking is a computational technique that predicts the binding affinity of ligands to receptor proteins.
* Acetylcholinesterase is a catalyzing enzyme found at postsynaptic neuromuscular junctions, especially in muscles and
nerves.
*QSAR algorithms correlate calculated molecular descriptors — like size, shape, surface area, solubility, flexibility, and polarity — to biological impacts within training datasets. Models can then predict potency, selectivity, and other properties for uncharacterized terpene derivatives.
By screening billions of hypothetical compounds in minutes, QSAR systems identify promising candidates for synthesis and testing. Eventually, tight integration of computer model predictions with laboratory validation may allow routine enhancement of any desired terpene attribute. QSAR modeling thus constitutes a critical tool in rational, optimized terpenoid therapeutics.
Absorption, Distribution, Metabolism, Excretion (ADME)
Following ingestion, topical, pulmonary or oral administration, terpene absorption, distribution, metabolism, excretion (ADME) processes mediate bioavailability and pharmacological activity. ADME processes.
Crucial pharmacokinetic measures include maximal concentration (Cmax), time to maximal concentration (Tmax), area under the curve (AUC), half-life (T1/2), steady-state volume of distribution (Vd), systemic clearance rates (CL), routes of biotransformation, and excretion proportions 21.
Tracking terpene disposition can help to guide future dosing strategies and multi- compound product development. State-of-the-art analytical platforms combining liquid chromatography, mass spectrometry, and isotope labeling now enable comprehensive ADME profiling of terpene metabolism after initial exposure.
High-resolution metabolomics data maps terpene breakdown cascades, bioaccumulation across organs, and kinetic excretion curves. Recently developed software models even assess metabolism in real-time using precision cut tissue slices22. Characterizing ADME will also help to optimize therapeutic indices for key terpenes.
Validation of Cannabis Chemovars
The term “chemovar” reflects the distinct chemical makeup of cannabis varieties defined by content of cannabinoids, terpenes, flavonoids and other specialized metabolites23.
However, most cannabis studies do not comprehensively analyze chemical profiles. As formulations containing multiple active components, medical cannabis products require validated chemovar standards ensuring accurate, reproducible therapeutic outcomes.
Sophisticated screens now enable high-throughput ultra-high-pressure liquid chromatography (UHPLC) quantification of terpene concentration. In tandem, optimized cultivation processing and curing strategies, together with manufacturing processes maximizing terpene stability promises truly personalized, precision cannabis medicine tailored to patient genetics and lifestyle.
Conclusion
Current scientific evidence overwhelmingly supports a whole plant approach in developing cannabis-based therapies, rather than isolated components. Individual cannabis constituents operate within a biological orchestra of cannabinoids, terpenes, flavonoids, and emerging compound classes.
Dynamic crosstalk between these players optimizes therapeutic outcomes above and beyond their atomistic effects. Indeed, research increasingly implicates terpenes as major conductors of this pharmacological symphony underlying cannabis synergies.
A more complete conceptual grasp of cannabis Entourage Effects is now within reach. Advanced analytical platforms promise comprehensive untargeted metabolomics mapping of cannabis chemovars, connecting distinct chemical profiles to empirical patient-reported outcomes.
In tandem, characterizing terpenoid pharmacodynamics through broad bioassay screening and activity-based protein profiling will uncover direct drug-target interactions and cell signaling impacts. State-of-the-art computational approaches facilitate virtual in silico experimentation on these rich datasets, efficiently optimizing experimental inquiries.
Integrating big data resources with rigorous wet lab hypothesis testing cycles will rapidly advance knowledge of how terpenes choreograph cellular systems-level changes. These insights may ultimately enable personalized, precision cannabis preparations with tunable terpene compositions ideal for patient genetics, lifestyles, priorities, and conditions. Such bespoke botanical medicines leverage eons of evolutionary plant innovation through biotechnology and analytic prowess.
The cannabis plant assuredly holds unique lessons for furthering human health — if we have the scientific tools, curiosity, and wisdom to listen. While the path ahead remains long, it promises a future where botanical remedies finally fulfill their rightful role in therapeutic synergy with conventional pharmaceutical options.
